Abstract
Background: Paroxysmal nocturnal hemoglobinuria (PNH) is an ultra-rare, acquired disorder caused by a mutation in the phosphatidylinositol glycan class A (PIGA) gene, which leads to impaired expression of complement-regulating proteins on the surface of hematopoietic cells. Clinical presentation of PNH includes hemolytic anemia, hemoglobinuria, and thrombosis. Complement component C5 inhibitors such as eculizumab are part of the current standard of care for patients with PNH; however, this is an intravenous treatment which relies on nurse administration. In addition, some patients experience an incomplete response to therapy, and may still experience breakthrough hemolytic events. Pozelimab and cemdisiran are investigational agents with a subcutaneous maintenance regimen that may be self-administered. Both agents act together to inhibit terminal complement through complementary mechanisms of action. Pozelimab is a fully human monoclonal antibody inhibitor of C5, while cemdisiran is an N-acetylgalactosamine-conjugated small interfering RNA (siRNA) that suppresses liver production of C5. The efficacy and safety of the combination of pozelimab and cemdisiran is being evaluated in an ongoing, phase 2, open-label single-arm study in patients with PNH who switch from eculizumab therapy (NCT04888507). The interim safety and preliminary efficacy results are presented here.
Methods: This ongoing phase 2 trial consists of a screening period (up to 42 days), a 32-week open-label treatment period (OLTP), an optional 52-week open-label extension period, and a 52-week post-treatment safety follow-up period. Patients transitioned from eculizumab therapy to the combination of pozelimab and cemdisiran over a 4-week period, and thereafter received the combination (pozelimab 400 mg and cemdisiran 200 mg) subcutaneously every 4 weeks.
Results: At the time of this interim data analysis (data cut-off March 18, 2022) six patients were enrolled and five patients were ongoing. While one patient discontinued after 29 days of treatment, the remaining five patients had a treatment duration of at least 169 days, with one completing the OLTP (Day 225).
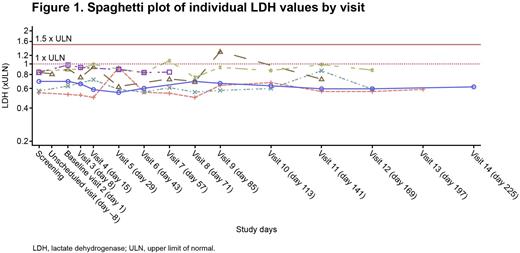
Up to the time of data cut-off, no patient had a lactate dehydrogenase (LDH) level greater than 1.5 x the upper limit of normal (ULN; Figure 1) or experienced an event of breakthrough hemolysis, including the two patients who were previously treated with higher doses of eculizumab (1200 mg and 1500 mg every 2 weeks, respectively). Furthermore, all but two LDH values remained normal (≤1.0 x ULN) at all timepoints evaluated. All patients achieved hemoglobin stabilization. CH50, a measure of terminal complement activity, remained fully suppressed at 0 kIU/L throughout the study up to the time of the data cut-off.
There were no serious or severe treatment emergent adverse events (TEAEs). Importantly, there were no meningococcal infections or adverse events due to potential large drug-target-drug immune complexes, or TEAEs leading to death, in this study. One patient discontinued study treatment due to two mild, non-serious TEAEs of headache at Days 2 and 16.
Conclusions: These interim results from a limited data set suggest that in patients with PNH transitioning from eculizumab treatment, including patients treated with higher than standard of care doses, the combination of pozelimab and cemdisiran was generally well tolerated, providing sustained control of intravascular hemolysis without any breakthrough hemolysis events. These findings support the ongoing development of pozelimab and cemdisiran combination therapy.
Disclosures
Kelly:Swedish Orphan Biovitrum AB: Membership on an entity's Board of Directors or advisory committees; Medscape: Other: educational work sponsored by Apellis with unrestricted grant paid to Medscape; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: conference support; Biocryst: Membership on an entity's Board of Directors or advisory committees; Sobi: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Biologix: Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Research Funding; Amgen: Consultancy. Munir:Janssen, AstraZeneca, Alexion, Abbvie, Novartis, Roche: Membership on an entity's Board of Directors or advisory committees; Janssen, AstraZeneca, Alexion, Sobi, Novartis, Roche, Abbvie, Gilead: Honoraria. Muus:Sobi: Other: travel support , Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees. Aurand:Regeneron Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Dain:Regeneron Pharmaceuticals, Inc.: Current Employment. Pavani:Regeneron Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Perlee:Regeneron: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Souttou:Regeneron Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Griffin:Alexion: Honoraria, Other: Conference support; BioCryst Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Sobi Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Medscape: Other: educational work sponsored by Apellis with unrestricted grant paid to Medscape.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal